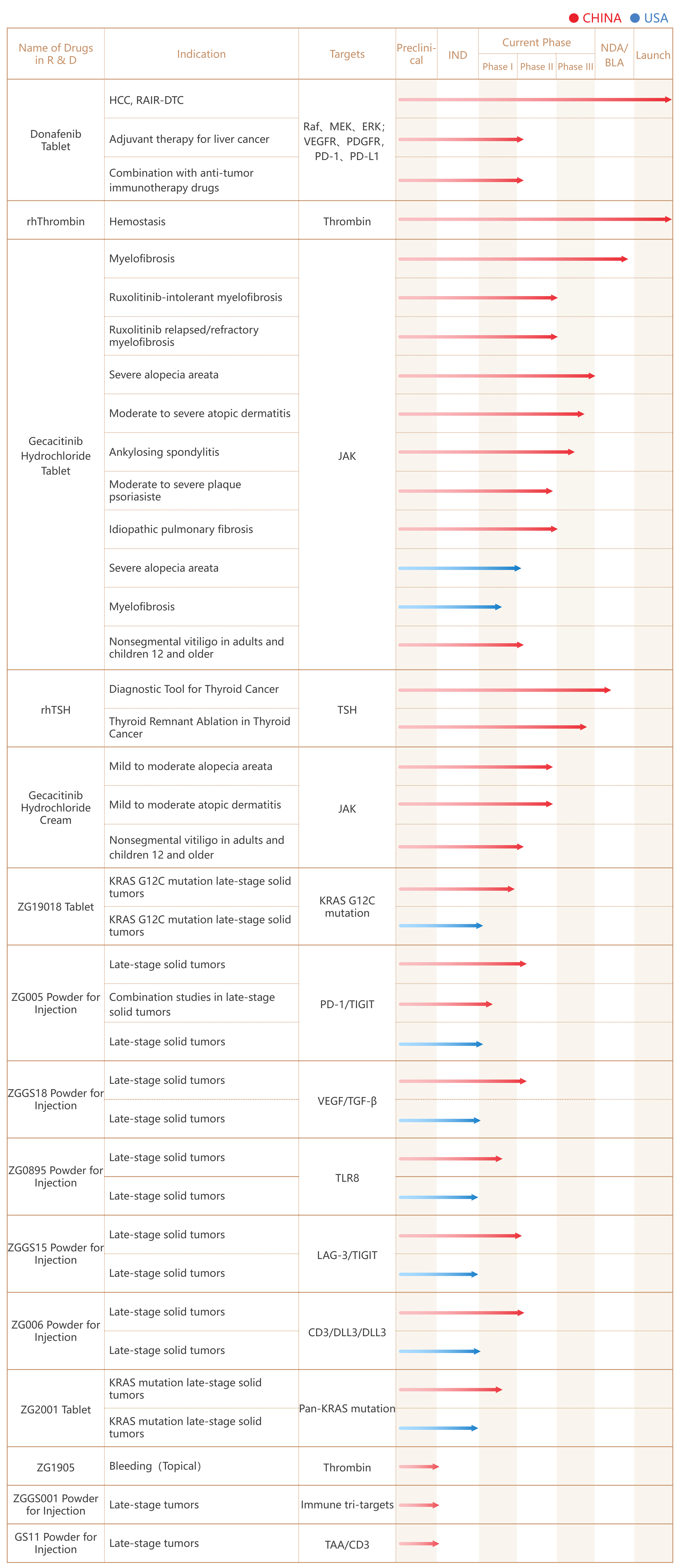
Except for Donafenib Tablet,Recombinant Human Thrombin,Jacktinib Tablet (used for the treatment of intermediate/high-risk myelofibrosis)has approved, the company has 13 major drug candidates, of which eight indications / two drug candidates (Jacktinib Tablet and Recombinant Human Thyroid-stimulating Hormone for Injection) are in the NDA, Phase III or registration clinical trial stages. Eight drug candidates (Jacktinib Cream, ZG19018 Tablet, ZG005 Powder For Injection, ZG006 Powder For Injection, ZGGS18 Powder For Injection, ZGGS15 Powder For Injection, ZG2001 Tablet and ZG0895 Powder For Injection) are under Phase I/II clinical trial stages.
 The Company is continually improving its R&D technology platforms to optimize and develop its pipeline through in-house R&D and external cooperation and prioritizing for product advancement, differentiation and commercialization. The company focuses on tumors, hemorrhagic and bleeding diseases and auto-immune diseases, and actively builds and strengthens its long-term competitiveness in the field of tumor, especially liver cancer treatment. Current clinical trials of the combination of its targeted small molecule drugs, including Donafenib, with immunotherapy antibody drugs like anti-PD-1/PD-L1 antibodies are going. Expanding upon this experience, the Company is developing new generation of immunotherapeutic antibody drugs and their combo-use. In addition, the company is developing a number of blood hemostasis products applicable to multiple hemorrhage conditions. Beyond a robust pipeline, the Company is also establishing a wide range of partnerships both in China and abroad that will greatly increase the geographical opportunities for our business.
The Company is continually improving its R&D technology platforms to optimize and develop its pipeline through in-house R&D and external cooperation and prioritizing for product advancement, differentiation and commercialization. The company focuses on tumors, hemorrhagic and bleeding diseases and auto-immune diseases, and actively builds and strengthens its long-term competitiveness in the field of tumor, especially liver cancer treatment. Current clinical trials of the combination of its targeted small molecule drugs, including Donafenib, with immunotherapy antibody drugs like anti-PD-1/PD-L1 antibodies are going. Expanding upon this experience, the Company is developing new generation of immunotherapeutic antibody drugs and their combo-use. In addition, the company is developing a number of blood hemostasis products applicable to multiple hemorrhage conditions. Beyond a robust pipeline, the Company is also establishing a wide range of partnerships both in China and abroad that will greatly increase the geographical opportunities for our business.
The Company's drugs currently in research and development are shown below: